
 |
|
Hydroponics - Indoor HorticultureHydroponics - Indoor Horticulture represents an educational, in-depth,
up-to-date,
indoor horticultural growers guide that covers all principles of indoor Hydroponics - Indoor Horticulture examines, explores, dissects and
presents a fully comprehensive step by step growers guide, relating
to all and every aspect of indoor hydroponic horticulture, with complete
chapters on plant biology, propagation, hydroponic systems, nutrients,
oxygen, carbon dioxide enrichment, pH, biological pest control, fungi/disease,
cuttings/clones, pruning/training, breeding, harvesting, equipment,
grow rooms, a full history of hydroponics, and more. |
| (Below follows a one page
sample taken from the book) Chapter 8 Water pH |
||
Water pH is a measure of the acidity or alkalinity of your water, be it in your system or out of your tap. This measure allows you to confirm whether the solution is too acidic for your plants, or too alkalinic; the measure reads from 0 to 14. Zero is the most acidic, 14 being its opposite and the most alkalinic, with 7 being neutral. The majority of nutrients used by a plant are soluble only within a limited range of acidity. This range is between 6 and 7 if you are growing in dirt, or 5.5 to 6.5 in most hydroponics systems to allow sufficient compensation for the particular grow medium used i.e. rockwool has a slightly higher alkalinity than clay pebbles, so when irrigating rockwool, you compensate for this by giving the medium a solution of around 5.5 on your pH level. In pebbles, it will be 6 to 6.5 for the same reason. Ok, should your reservoir become too acidic then the dissolved nutrients in the water will precipitate and you will get nutrient lock, therefore, the necessary nourishment will be unavailable to the plants. This is also the same if the solution gets too alkalinic. Tests have shown that a plant subjected to conditions where the pH is too low typically end up very small and only growing, if at all, at a snail’s pace. The same tests have shown that plants subjected to high pH are very pale with also the same slow, stunted growth characteristics. pH can be measured using simple colour changing chemicals such as litmus paper or more accurately measured using a digital pH meter available from good hydroponics or aquarium retailers. All water has a pH value which can be measured using the above. After adding nutrients to your reservoir, you should remember that the nutrient employed will affect and change the level of pH in the reservoir, so always stir the reservoir thoroughly after adding nutrients and then allow it to rest before testing the pH and adjusting the level in your reservoir. If you find the level of pH is too high in your system then you should adjust it with some pH down which typically consists of phosphoric acid at 81% and is again available at your local hydro store. You should only employ a tiny drop at a time, then stir and re-test before adding any more as this stuff is extremely concentrated and takes a little time to mix properly. If, on the other hand, you find that the solution is too acidic, then you need to employ some pH up – this is usually potassium hydroxide at 50% and again available at your local hydroponics store. Like the pH down, use very sparingly and mix it well before re-testing. Never mix these two solutions directly as these two chemicals are diametrically opposed and when mixed together as a concentrate, they will conflict and possibly explode. It is also worth noting that if you put too much pH down in, it is not good practice to readjust it with pH up as both of these chemicals, when heavily employed, knock out very important food chains, so the more you use, the worse it will be for your plants as more good nutrients in the reservoir are destroyed. So if you get it wrong, simply throw the stock solution away and start again, being more careful and don’t overdo it this time. On this note it is once more worth |
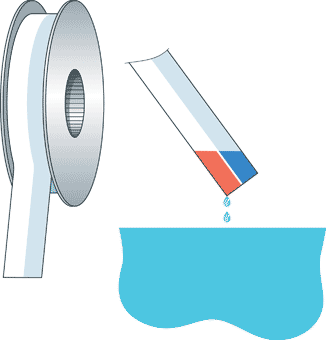 Example of Litmus pH paper
Example of Litmus pH papermentioning that if you are running a re-circulating hydroponics system, like most growers in the UK, then you should be aware that in hard water areas where you have to employ much more pH down to get the pH of the water to the right levels, that it is not advisable to dump nutrients on a regular basis, as every time you do this, you not only need to fill the reservoir with more fresh water and nutrients but you also have to use a lot of pH down on every occasion. In so doing, you are knocking out and destroying food chains that the plants want as you have to use a lot of pH down to get the solution to the correct level. On the contrary, when you simply top up an existing reservoir with fresh water, do a little bit of pH balancing and the food chain is hardly affected. So, by replacing the solution in the reservoirs, you think you are giving your plants fresh and therefore more food when in reality, because you need to add so much pH down as the solution is not already buffered, you are in fact destroying much of the usable food chains. However, if you were simply to top up and replace the used water and nutrients, then readjust the pH, you will actually be helping your plants out more as not many food chains are lost in this process. It is advised on new systems to check your pH daily, and once the system has been broken in and buffered, then every 2-4 days should suffice. Plant growth fluctuates the pH in a reservoir as they deplete differing nutrients from the reservoir, so bear this in mind. Also, tap water over periods of time also fluctuates in pH levels, so do not always think that your tap water is a particular level of pH, as some days it is not. If possible, it is also advised to test the pH of the water inside the actual growing medium by using a syringe or the likes, as sometimes the level of the pH in the growing area of the roots can be different to that in the reservoir. If you find that it is different, then you should counterbalance this difference by adjusting the reservoir accordingly. That is to say, if you find that the solution inside the rockwool for example has a pH of 6.5 however, your reservoir’s pH is reading 6, and you actually want the water inside the rockwool to read 6, then you should adjust the reservoir down to 5.5 in order to achieve this. |
|
|
 |